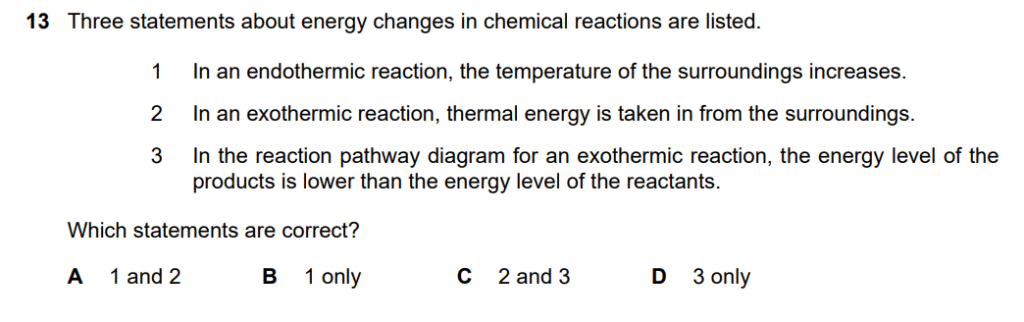
The correct answer is D, which states that only statement 3 is correct.
- Statement 1 is incorrect. An endothermic reaction absorbs thermal energy from the surroundings. This causes the temperature of the surroundings to decrease, not increase.
- Statement 2 is incorrect. An exothermic reaction releases thermal energy into the surroundings. This means thermal energy is given out, not taken in.
- Statement 3 is correct. In an exothermic reaction, the chemical bonds in the reactants have more energy than the bonds in the products. The excess energy is released as heat. Therefore, on a reaction pathway diagram, the energy level of the products is lower than the energy level of the reactants.
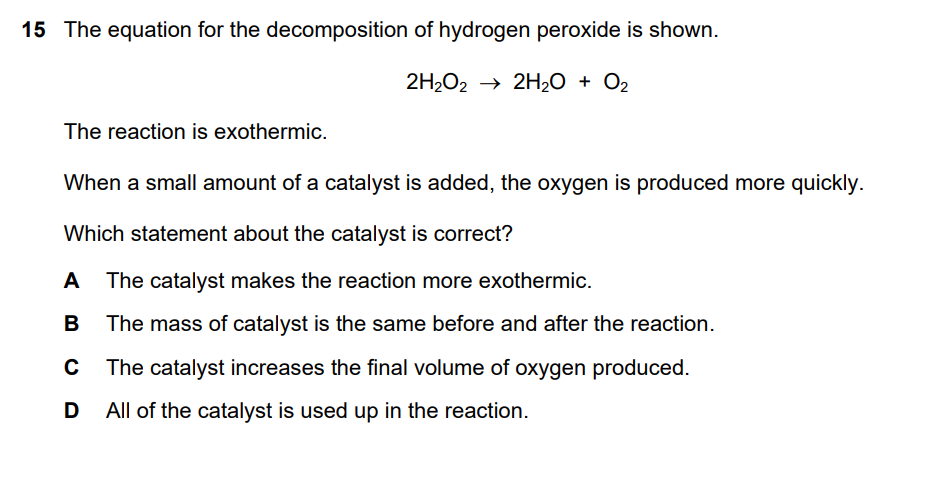
A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction itself. Catalysts work by providing an alternative reaction pathway with a lower activation energy. This allows a greater proportion of reactant particles to have enough energy to react, thus speeding up the process.
Let’s analyze each statement:
- A. The catalyst makes the reaction more exothermic. This is incorrect. A catalyst does not affect the overall energy change of a reaction (ΔH). It only affects the reaction rate. The initial and final energy levels of the reactants and products remain the same.
- B. The mass of catalyst is the same before and after the reaction. This is correct. By definition, a catalyst is not consumed in the reaction. It can be recovered and reused, meaning its mass remains constant.
- C. The catalyst increases the final volume of oxygen produced. This is incorrect. The final amount of product is determined by the amount of the limiting reactant, which in this case is hydrogen peroxide (H2O2). A catalyst only increases the rate at which the products are formed; it does not change the total amount produced.
- D. All of the catalyst is used up in the reaction. This is incorrect. This is the opposite of a catalyst’s key property. A catalyst is not used up in the reaction.
