1. Understanding Atoms and Elements
- Every substance is made of atoms (smallest particles of an element).
- Atoms can lose or gain electrons during reactions, forming ions.
- Metals usually lose electrons, while nonmetals usually gain electrons.
2. Concept of Electrons
- Electrons are negatively charged particles that move around the nucleus.
- In chemical reactions, changes in electron number or position lead to oxidation or reduction.
- Oxidation and reduction always happen together (in pairs).
3. Ions and Charges
- When an atom loses electrons, it becomes a positive ion (cation).
- When it gains electrons, it becomes a negative ion (anion).
- This electron movement is the base of oxidation and reduction.
4. Chemical Reactions Involving Oxygen
- Early definitions of oxidation and reduction were based on oxygen:
- Oxidation: Addition of oxygen or removal of hydrogen.
- Reduction: Removal of oxygen or addition of hydrogen.
- Students must know oxygen and hydrogen both can cause such reactions.
5. Electron Transfer View
- In modern chemistry:
- Oxidation means loss of electrons.
- Reduction means gain of electrons.
- Remember with OIL RIG: Oxidation Is Loss, Reduction Is Gain (of electrons).
6. Oxidizing and Reducing Agents
- Oxidizing agent: Causes oxidation by taking electrons (it gets reduced).
- Reducing agent: Causes reduction by giving electrons (it gets oxidized).
- Always think: the one that gains electrons is reduced; the one that loses electrons is oxidized.
7. Reaction Pairing
- Oxidation and reduction always occur together in a reaction.
If one substance is oxidized, another must be reduced.
8. Oxidation Numbers (States)
- Each atom has an oxidation number showing how many electrons it has gained or lost.
- A change in oxidation number during a reaction shows oxidation (increase) or reduction (decrease).
9. Examples to Recognize
- Oxidation example:
Mg → Mg²⁺ + 2e⁻ (loss of electrons) - Reduction example:
Cl₂ + 2e⁻ → 2Cl⁻ (gain of electrons)
10. Energy and Real-life Examples
- Oxidation-reduction reactions (redox reactions) are common in daily life:
- Rusting of iron
- Burning of fuels
- Respiration in living things
- Photosynthesis in plants
- Batteries and electrolysis
Another Definition for Oxidation and Reduction
When magnesium burns in oxygen, magnesium oxide is formed:
2Mg (s) + O₂ (g) → 2MgO (s)
The magnesium has clearly been oxidized.
Oxidation and reduction always take place together, so the oxygen must have been reduced.
But how?
Let’s see what is happening to the electrons.
During the reaction, each magnesium atom loses two electrons, and each oxygen atom gains two electrons.
This leads us to a new definition:
If a substance loses electrons during a reaction, it has been oxidized.
If it gains electrons, it has been reduced.
The reaction is called a redox reaction.
Here’s the same text rewritten clearly and neatly:
Writing Half-Equations to Show the Electron Transfer
You can use half-equations to show how electrons are transferred in a reaction.
One half-equation shows electron loss (oxidation), and the other shows electron gain (reduction).
Here is how to write the half-equations for the reaction:
1. Write down each reactant, with the electrons it gains or loses.
Magnesium:
Mg → Mg²⁺ + 2e⁻
Oxygen:
O + 2e⁻ → O²⁻
2. Check that each substance is in its correct form (ion, atom, or molecule) on each side of the arrow. If it is not, correct it.
Oxygen is not in its correct form on the left side because it exists as a molecule (O₂).
So, change O to O₂.
You must also double the number of electrons and oxide ions.
Oxygen:
O₂ + 4e⁻ → 2O²⁻
3. The number of electrons must be the same in both equations.
If not, multiply one or both equations to make them equal.
Multiply the magnesium half-equation by 2:
Magnesium:
2Mg → 2Mg²⁺ + 4e⁻
Now both equations have 4 electrons:
Magnesium: 2Mg → 2Mg²⁺ + 4e⁻
Oxygen: O₂ + 4e⁻ → 2O²⁻
The equations are now balanced, each with 4 electrons.
Redox without Oxygen
Our definition of redox reactions is now much broader:
Any reaction in which electron transfer takes place is a redox reaction.
So, the reaction does not have to include oxygen. Look at these examples:
1. The Reaction Between Sodium and Chlorine
The equation is:
2Na (s) + Cl₂ (g) → 2NaCl (s)
The sodium atoms give electrons to the chlorine atoms, forming ions as shown on the right.
So, sodium is oxidized, and chlorine is reduced.
This means the reaction is a redox reaction.
Look at the half-equations:
- Sodium: 2Na → 2Na⁺ + 2e⁻ (oxidation)
- Chlorine: Cl₂ + 2e⁻ → 2Cl⁻ (reduction)
2. The Reaction Between Chlorine and Potassium Bromide
When chlorine gas is bubbled through a colorless solution of potassium bromide,
the solution turns orange due to this reaction:
Cl₂ (g) + 2KBr (aq) → 2KCl (aq) + Br₂ (aq)
Colorless → Orange
Bromine has been displaced.
The half-equations for the reaction are:
- Chlorine: Cl₂ + 2e⁻ → 2Cl⁻ (reduction)
- Bromide ion: 2Br⁻ → Br₂ + 2e⁻ (oxidation)
From Half-Equations to the Ionic Equation
Adding the balanced half-equations gives the ionic equation for the reaction.
An ionic equation shows the ions that take part in the reaction.
For example, for the reaction between chlorine and potassium bromide:
Cl₂ + 2e⁻ → 2Cl⁻
2Br⁻ → Br₂ + 2e⁻
The electrons cancel out, giving the ionic equation:
Cl₂ + 2Br⁻ → 2Cl⁻ + Br₂
Redox: A Summary
- Oxidation is gain of oxygen or loss of electrons.
- Reduction is loss of oxygen or gain of electrons.
- Oxidation and reduction always take place together in a redox reaction.

To solve this, let’s carefully analyze each of the four statements about the reaction between a Group I metal (like lithium, sodium, or potassium) and cold water:
1. The metal melts:
- This is a physical change because the metal transitions from a solid state to a liquid state due to the heat produced in the exothermic reaction. The chemical composition of the metal remains the same; only the state changes.
2. Hydrogen is produced:
- This is a chemical change because it involves the production of hydrogen gas (H₂) from the reaction between the metal and water. A new substance (hydrogen gas) is formed.
3. Steam is produced:
- This is a physical change because steam is simply water in a gaseous state. The water changes its state from liquid to gas due to the heat produced during the reaction. The chemical composition of water doesn’t change; only the state changes.
4. The pH of the solution increases:
- This is a chemical change because it results from the formation of a metal hydroxide (e.g., lithium hydroxide, NaOH) in water. This increases the pH of the solution, making it more basic.
Now, let’s match the statements with the physical changes:
- Physical changes: 1 (metal melts) and 3 (steam is produced).
Thus, the correct answer is A. 1 and 3.
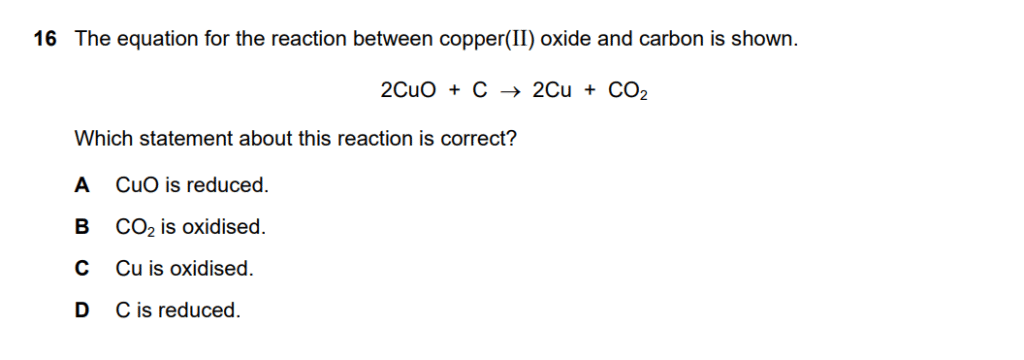
Fundamental concepts:
- Oxidation: Loss of electrons, increase in oxidation state
- Reduction: Gain of electrons, decrease in oxidation state
- Oxidizing agent: Causes oxidation, gets reduced itself
- Reducing agent: Causes reduction, gets oxidized itself
Memory aid: “OIL RIG”
- Oxidation Is Loss (of electrons)
- Reduction Is Gain (of electrons)
Analyzing the Reaction
Given equation: 2CuO + C → 2Cu + CO₂
Step-by-Step Oxidation State Analysis
Step 1: Determine Oxidation States Before Reaction
CuO (Copper(II) oxide):
- Cu: +2 (given as copper(II))
- O: -2 (oxygen typically -2 in compounds)
C (Carbon):
- C: 0 (elemental form)
Step 2: Determine Oxidation States After Reaction
Cu (Copper metal):
- Cu: 0 (elemental form)
CO₂ (Carbon dioxide):
- C: +4 (since O is -2, and molecule is neutral: C + 2(-2) = 0, so C = +4)
- O: -2
Step 3: Identify Changes in Oxidation States
Copper changes:
- Cu: +2 → 0 (decrease of 2)
- This is REDUCTION (oxidation state decreases)
Carbon changes:
- C: 0 → +4 (increase of 4)
- This is OXIDATION (oxidation state increases)
Step 4: Analyze Each Option
A – CuO is reduced:
- CuO contains Cu²⁺ which goes to Cu⁰
- Oxidation state decreases (+2 → 0)
- CORRECT – CuO (specifically the Cu²⁺ ion) is reduced
B – CO₂ is oxidised:
- CO₂ is a product, not a reactant being oxidized
- The carbon in CO₂ has already been oxidized (from C⁰ to C⁴⁺)
- INCORRECT – CO₂ is the product of oxidation, not what gets oxidized
C – Cu is oxidised:
- Cu (metal) is a product with oxidation state 0
- The Cu²⁺ in CuO was actually reduced to form Cu⁰
- INCORRECT – Cu metal is the reduced product
D – C is reduced:
- Carbon goes from 0 to +4 oxidation state
- This is an increase, which means oxidation, not reduction
INCORRECT – C is oxidized, not reduced
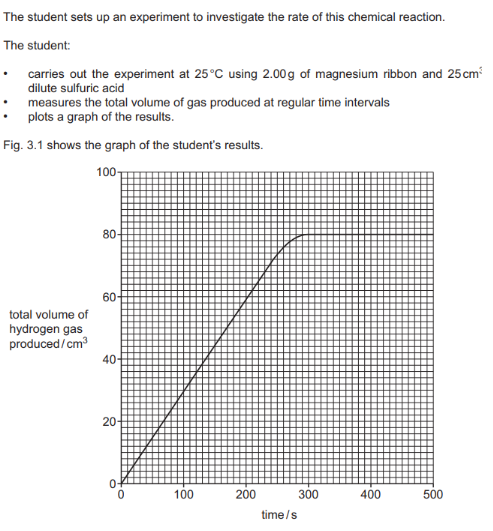
The word equation for the reaction of magnesium with dilute sulfuric acid is: Magnesium + Sulfuric Acid -> Magnesium Sulfate + Hydrogen.
Deduce the total volume of hydrogen gas produced in 100s of reaction :
Why reaction stops at 300s : Due to limiting reactant
The reaction is repeated at higher temperature
When the experiment is repeated at a higher temperature, the reaction rate will increase. This means the reaction will be faster, producing the same total volume of gas in less time. The line on the graph should start from the origin, have a steeper initial slop
The reaction is repeated using Sulphuric acid at low concentration :
All other conditions remain same
Describe how the rate of reaction differ when sulphuric acid of low concentration is used :
When sulfuric acid of a lower concentration is used, the rate of reaction will decrease. A lower concentration means there are fewer reactant particles per unit volume, leading to less frequent collisions between reactant particles and thus a slower reaction rate.
The reaction is done using a catalyst
All the other conditions remain same
Describe how rate of reaction differ when catalyst is used :
When a catalyst is used, the rate of reaction will increase. A catalyst provides an alternative reaction pathway with a lower activation energy, increasing the frequency of successful collisions without being consumed in the reaction.
Describe test for Hydrogen
Test: Place a lit splint near the mouth of a test tube containing the gas.
Observations: A squeaky pop sound is heard. This is due to the rapid combustion of hydrogen gas in the presence of oxygen.
