Understanding Atoms – The Building Blocks of Matter
What Are Atoms?
Key Definition: Atoms are the smallest particles of matter that cannot be broken down further by chemical means.
Critical Atomic Concepts
1. Atomic Scale and Size
- Incredibly small: A million sodium atoms could fit across a full stop
- Invisible to naked eye: Single atoms are far too small to see
- Observable only in groups: We can only see elements when billions of atoms are present together
2. Atomic Structure Overview
- Mostly empty space: Atoms consist primarily of empty space
- Central nucleus: Dense core containing protons and neutrons
- Electron cloud: Negatively charged particles that move around the nucleus
- Scale analogy: If an atom were the size of a football stadium, the nucleus would be the size of a pea
Elements – Pure Substances Made of One Type of Atom
Element Definition and Properties
An element contains only one kind of atom.
Key Element Facts
- Natural occurrence: Around 90 elements found in Earth and atmosphere
- Artificial elements: Scientists have created nearly 50 additional elements in laboratories
- Instability of artificial elements: Many artificial elements are very unstable and last only seconds before breaking down
- Why artificial elements aren’t found in nature: They break down too quickly to exist naturally
Element Examples from Images
- Sodium (Na): Made of tiny sodium atoms – appears as silvery white chunks
- Carbon (C): Diamond form made of carbon atoms arranged in crystal structure
- Mercury (Hg): Liquid metal made of mercury atoms – highly reflective
Element Symbols and Naming
Chemical Symbols System
Purpose: Each element has a unique symbol to make identification easy
Symbol Rules and Examples
- Single letter symbols: Some elements use one letter (rare)
- Two letter symbols: Most common format – first letter capitalized, second lowercase
- Latin origins: Many symbols come from Latin names
- Carbon = C (from Latin “carbonum”)
- Potassium = K (from Latin “kalium”)
- Sodium = Na (from Latin “natrium”)
Historical Naming
- Discoverer names: Some elements named after people who discovered them
- Example from image: Phosphorus discovered by Hennig Brand in 1669
- Special property: Phosphorus glows in the dark (chemiluminescence)
The Periodic Table – Organizing the Elements
Periodic Table Structure
The Periodic Table gives names and symbols for all elements and organizes them by properties.
Table Organization
Columns (Groups):
- Numbered I, II, III… or 1, 2, 3…
- Elements in same group: Have similar properties
- Predictable behavior: If you know how one Group I element behaves, you can predict others
Rows (Periods):
- Horizontal rows across the table
- Property changes: As you move across a period, properties change gradually
Metal vs. Non-Metal Organization
- Zig-zag line separates metals from non-metals
- Metals: Located on the left side of the line
- Non-metals: Located on the right side of the line
- Exception: Hydrogen is a non-metal but placed with Group I
- Transition across period: Change from metal to non-metal properties
Atomic Numbers on Periodic Table
Small numbers beside each symbol tell us about the atom’s structure
Sub-Atomic Particles – Inside the Atom
Three Types of Particles
| Particle | Location | Mass (atomic mass units) | Charge |
|---|---|---|---|
| Proton | Nucleus | 1 unit | Positive (+1) |
| Neutron | Nucleus | 1 unit | None (neutral) |
| Electron | Electron shells around nucleus | Almost nothing (≈0) | Negative (-1) |
Mass Considerations
- Protons and neutrons: Have significant mass (1 atomic mass unit each)
- Electrons: Mass is so small it’s usually taken as zero
- Atomic mass units: Used because particles are too light to measure in grams
- Why electrons ignored: Their mass is negligible compared to protons and neutrons
Atomic Structure Arrangement
Nucleus Structure
- Central location: Protons and neutrons cluster together in the center
- Heavy part of atom: Contains virtually all the atom’s mass
- Very small size: Extremely tiny compared to overall atom size
Electron Arrangement
- Electron shells: Electrons occupy different energy levels called shells
- Rapid movement: Electrons circle very fast around the nucleus
- Different energy levels: Electrons exist at various distances from nucleus
- Shell model: Electrons arranged in concentric circles around nucleus
Example: Sodium Atom Structure
- 11 protons in the nucleus (determines element identity)
- 11 electrons around the nucleus (equals number of protons)
- 12 neutrons in the nucleus (mass number – proton number)
- Arrangement: Electrons distributed in shells: 2, 8, 1
Proton Number (Atomic Number)
Defining Element Identity
The proton number identifies what element an atom is.
Key Principles
- Unique identifier: Only sodium atoms have 11 protons
- Different elements: Every other element has a different proton number
- Cannot change: If you change the proton number, you get a different element
- Atomic number: The proton number is called the atom’s atomic number
Examples
- Sodium: Proton number = 11 (always)
- Carbon: Proton number = 6 (always)
- Oxygen: Proton number = 8 (always)
Electron Number and Atomic Charge
Electron-Proton Relationship
Every atom has an equal number of protons and electrons.
Charge Balance Calculation
Using Sodium Example:
- 11 protons: Each with +1 charge = +11 total positive charge
- 11 electrons: Each with -1 charge = -11 total negative charge
- Net result: +11 + (-11) = 0
- Overall charge: Atoms have no overall charge (neutral)
Universal Rule
All atoms have no overall charge because:
- Positive charges from protons
- Equal negative charges from electrons
- Charges cancel each other out perfectly
Practical Applications and Real-World Examples
Element Extraction and Use
- Sulfur collection: Harvested from volcanic craters in Indonesia
- Cosmetic industry: Sulfur used as ingredient in many cosmetic products
- Historical significance: Elements like phosphorus discovered through experimental work
Why This Knowledge Matters
- Chemical reactions: Understanding atomic structure explains how elements react
- Material properties: Atomic arrangement determines physical properties
- Industrial applications: Knowledge helps in element extraction and purification
- Medical applications: Atomic structure affects how elements interact with living tissue
Common Misconceptions and Exam Tips
Important Clarifications
❌ Wrong: “Atoms are solid particles like tiny marbles” ✅ Correct: “Atoms are mostly empty space with a tiny, dense nucleus”
❌ Wrong: “Electrons orbit like planets around the sun”
✅ Correct: “Electrons exist in probability clouds at different energy levels”
❌ Wrong: “All atoms of different elements look different” ✅ Correct: “Atoms are too small to see; we identify elements by their properties”
Extension Concepts for Higher Achievement
Isotopes Introduction
- Same element, different mass: Atoms with same proton number but different neutron numbers
- Why mass can vary: Neutron number can change without changing element identity
- Example: All carbon atoms have 6 protons, but some have 6, 7, or 8 neutrons
Ion Formation Preview
- Gaining/losing electrons: Atoms can gain or lose electrons to form charged particles
- Charge imbalance: Unequal protons and electrons create overall charge
- Chemical bonding: Understanding atomic structure explains how atoms combine
Electron Energy Levels
- Quantized energy: Electrons can only exist at specific energy levels
- Shell filling rules: Electrons fill inner shells before outer shells
- Chemical reactivity: Outer electron arrangements determine how elements react
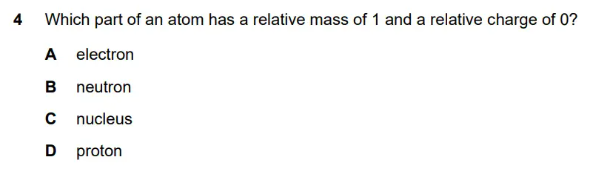
| Particle | Relative Mass | Relative Charge | Location |
|---|---|---|---|
| Proton | 1 | +1 | Nucleus |
| Neutron | 1 | 0 | Nucleus |
| Electron | 1/1836 (≈0) | -1 | Electron shells |

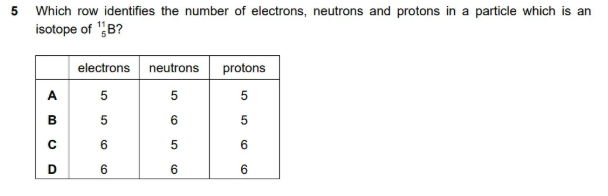
- Mass number (A) = 11
- Atomic number (Z) = 5
- Element = Boron (B)
Protons = Atomic number = 5
Neutrons = Mass number – Atomic number Neutrons = 11 – 5 = 6

The correct statement is 2 only. Let’s break down each option.
- Statement 1 is incorrect. Potassium iodide (KI) is an ionic compound. It is formed from a potassium cation (K+) and an iodide anion (I−). Anions are negatively charged ions, and cations are positively charged.
- Statement 2 is correct. Ionic compounds like potassium iodide conduct electricity when their ions are free to move. This happens when the compound is in a molten state or dissolved in a solvent like water, forming an aqueous solution. In the solid state, the ions are locked in a crystal lattice and cannot move.
- Statement 3 is incorrect. Potassium iodide is an ionic compound, not a covalent one. This means that potassium transfers an electron to iodine, forming ions. They don’t share electrons. Sharing of electrons occurs in covalent compounds.

1. Argon (Ar)
- Argon is a noble gas → atoms are stable, with a full outer shell.
- No bonding, no shared electrons.
✅ Not correct.
2. Methane (CH₄)
- Carbon shares electrons with 4 hydrogens → 4 covalent bonds (shared pairs).
✅ Yes, contains shared pairs.
3. Iron(III) oxide (Fe₂O₃)
- This is an ionic compound: Fe³⁺ and O²⁻ ions held by electrostatic attraction.
- No shared pairs, just transfer of electrons.
❌ Not correct.
4. Chlorine (Cl₂)
- A molecule of chlorine: two Cl atoms share a pair of electrons → covalent bond.
✅ Yes, contains shared pairs.
✔ Answer:
The substances with shared pairs are 2 (methane) and 4 (chlorine).
Correct option = D (2 and 4)
Ionic Bond = Metal + Non-Metal
Covalent Bond = Non-Metal + Non-Metal

- Diamond is an allotrope of carbon with a giant covalent structure. Each carbon atom is bonded to four other carbon atoms in a rigid, three-dimensional tetrahedral lattice. This strong, extensive network of bonds makes diamond extremely hard and gives it a very high melting point.
- Ammonia (NH3) and Carbon Dioxide (CO2) have simple molecular structures. The atoms within each molecule are held by strong covalent bonds, but the individual molecules are only held together by weak intermolecular forces. This is why they exist as gases at room temperature.
- Water (H2O) also has a simple molecular structure. While the molecules are attracted to each other by strong hydrogen bonds, the overall structure is not a giant covalent lattice.
