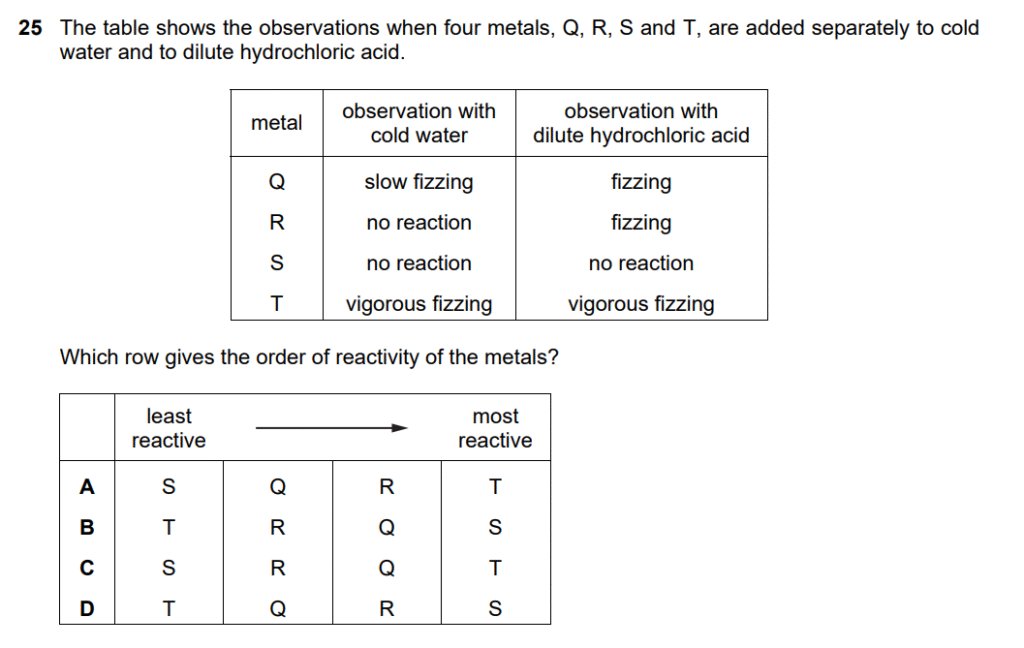
Key principle: More reactive metals react more vigorously with both water and acids.
Analyzing each metal:
Metal T:
- Vigorous fizzing with cold water
- Vigorous fizzing with dilute HCl
- Most reactive (reacts vigorously with both)
Metal Q:
- Slow fizzing with cold water
- Fizzing with dilute HCl
- Moderately reactive (reacts with both, but slower with water)
Metal R:
- No reaction with cold water
- Fizzing with dilute HCl
- Less reactive (only reacts with acid, not water)
Metal S:
- No reaction with cold water
- No reaction with dilute HCl
- Least reactive (doesn’t react with either)
Reactivity order (least → most reactive):S < R < Q < T
Checking options:
- A: S, Q, R, T ✗ (wrong order of Q and R)
- B: T, R, Q, S ✗ (completely reversed)
- C: S, R, Q, T ✓ (matches our analysis)
- D: T, Q, R, S ✗ (reversed order)
Answer: C
