Properties of Solids, Liquids and Gases
SOLIDS
Key Properties:
- Fixed shape and volume – particles are locked in position
- High density – particles closely packed together
- Incompressible – very little space between particles
- Do not flow – particles cannot move past each other
- Low kinetic energy – particles vibrate around fixed positions
Particle Arrangement:
- Particles arranged in regular, repeating patterns (crystal lattice)
- Strong intermolecular forces hold particles in fixed positions
- Particles vibrate but cannot translate (move from place to place)
LIQUIDS
Key Properties:
- Fixed volume but variable shape – takes shape of container
- Medium density – particles close but can slide past each other
- Nearly incompressible – small spaces between particles
- Can flow – particles can move past each other
- Medium kinetic energy – particles can translate and rotate
Particle Arrangement:
- Particles close together but not in fixed positions
- Intermolecular forces present but weaker than in solids
- Particles can slide past each other while maintaining proximity
GASES
Key Properties:
- Variable shape and volume – completely fills container
- Low density – particles far apart
- Highly compressible – large spaces between particles
- Flow easily – particles move freely and independently
- High kinetic energy – particles move rapidly in all directions
Particle Arrangement:
- Particles widely separated with random motion
- Weak intermolecular forces (negligible at normal conditions)
- Particles move independently in straight lines until collisions occur
Particle Separation Analysis
| State | Relative Particle Separation | Force Between Particles | Motion Type |
|---|---|---|---|
| Solid | Very close (touching) | Very strong | Vibrational only |
| Liquid | Close (nearly touching) | Moderate | Vibrational + translational |
| Gas | Far apart | Very weak/negligible | Rapid, random motion |
Changes of State
State Change Processes
MELTING (Solid → Liquid)
- Energy requirement: Heat energy input needed
- Particle behavior: Increased vibration breaks some intermolecular bonds
- Temperature: Occurs at specific melting point
- Volume change: Slight increase in volume
BOILING (Liquid → Gas)
- Energy requirement: Significant heat energy input
- Particle behavior: Particles gain enough energy to overcome all intermolecular forces
- Temperature: Occurs at specific boiling point
- Volume change: Dramatic increase in volume
EVAPORATING (Liquid → Gas at surface)
- Energy requirement: Occurs at temperatures below boiling point
- Particle behavior: Surface particles with highest kinetic energy escape
- Temperature: Occurs at any temperature
- Rate factors: Temperature, surface area, air movement, humidity
FREEZING/SOLIDIFYING (Liquid → Solid)
- Energy change: Heat energy released
- Particle behavior: Reduced motion allows intermolecular forces to lock particles
- Temperature: Occurs at freezing point (same as melting point)
CONDENSING (Gas → Liquid)
- Energy change: Heat energy released
- Particle behavior: Reduced kinetic energy allows intermolecular forces to act
- Temperature: Occurs at condensation point (same as boiling point)
SUBLIMING (Solid → Gas directly)
- Examples: Dry ice (solid CO₂), iodine crystals
- Energy requirement: High energy input needed
- Particle behavior: Particles go directly from fixed positions to free motion
Effects of Temperature and Pressure on Gas Volume
Temperature Effects (Charles’s Law)
At constant pressure:
- Heating: Volume increases proportionally
- Cooling: Volume decreases proportionally
- Relationship: V ∝ T (in Kelvin)
- Explanation: Higher temperature → higher kinetic energy → particles move faster and spread out more
Pressure Effects (Boyle’s Law)
At constant temperature:
- Increased pressure: Volume decreases
- Decreased pressure: Volume increases
- Relationship: P ∝ 1/V (inverse relationship)
- Explanation: Higher pressure forces particles closer together
Combined Gas Law
For a fixed amount of gas: PV/T = constant
5. Kinetic Particle Theory and State Changes
Core Principles of Kinetic Theory
- All matter consists of tiny particles in constant motion
- Temperature is proportional to average kinetic energy of particles
- Intermolecular forces exist between particles
- Collisions between particles are elastic (no energy lost)
Energy Changes During State Transitions
HEATING CURVES:
- Sloped sections: Temperature rises as kinetic energy increases
- Flat sections: Temperature constant as potential energy changes (bonds breaking/forming)
- Melting plateau: Energy used to break intermolecular bonds
- Boiling plateau: Energy used to completely separate particles
COOLING CURVES:
- Reverse process: Energy released as bonds form
- Crystallization: Particles arrange into ordered structure
- Condensation: Gas particles lose energy and come together
6. Kinetic Theory and Gas Behavior
Temperature Effects on Gases
Molecular Level Explanation:
- Higher temperature = higher average kinetic energy
- Particles move faster and collide with container walls more frequently
- More forceful collisions create higher pressure
- If pressure kept constant, volume must increase to maintain equilibrium
Pressure Effects on Gases
Molecular Level Explanation:
- Higher pressure forces particles closer together
- Reduced volume means particles have less space to move
- Same number of particles in smaller space increases collision frequency
- Temperature remains constant so average kinetic energy unchanged
Gas Laws from Kinetic Theory
- Boyle’s Law (P₁V₁ = P₂V₂): Pressure and volume inversely related
- Charles’s Law (V₁/T₁ = V₂/T₂): Volume and temperature directly related
- Gay-Lussac’s Law (P₁/T₁ = P₂/T₂): Pressure and temperature directly related
1. Definition and Explanation of Diffusion
What is Diffusion?
Definition: The net movement of particles from a region of high concentration to a region of low concentration, down a concentration gradient, as a result of their random kinetic motion.
Key Characteristics of Diffusion
- Spontaneous process – occurs without external energy input
- Random particle movement – individual particles move in all directions
- Net movement – overall flow is from high to low concentration
- Continues until equilibrium – stops when concentration is uniform
- Passive process – driven by kinetic energy particles already possess
Kinetic Particle Theory Explanation
Particle Movement Principles
- Random Motion: All particles are in constant, random motion due to kinetic energy
- Collision Effects: Particles collide with each other and change direction randomly
- Concentration Gradients: More particles in high concentration regions means more random movements out of that region
- Statistical Probability: More particles moving out of high concentration areas than moving in
Step-by-Step Diffusion Process
- Initial State: Particles concentrated in one region
- Random Movement Begins: Particles move in all directions due to kinetic energy
- Net Migration: More particles leave high concentration area than enter it
- Spreading Continues: Particles continue to spread throughout available space
- Equilibrium Reached: Uniform distribution achieved – no net movement
Diffusion in Different States of Matter
GASES – Fastest Diffusion
Why gases diffuse quickly:
- Large spaces between particles – less obstruction to movement
- High kinetic energy – particles move rapidly
- Weak intermolecular forces – particles move independently
- No fixed structure – particles can move in any direction
Examples:
- Perfume spreading through a room
- Gas leak detection
- Mixing of different gases in the atmosphere
LIQUIDS – Moderate Diffusion
Why liquids diffuse more slowly than gases:
- Particles closer together – more obstacles to movement
- Lower kinetic energy than gases – slower particle movement
- Stronger intermolecular forces – particles influence each other’s movement
- Some structure – particles must squeeze past each other
Examples:
- Food coloring spreading in water
- Sugar dissolving in tea
- Ink dispersing in water
SOLIDS – Slowest Diffusion
Why diffusion in solids is extremely slow:
- Particles very close together – maximum obstruction
- Lowest kinetic energy – particles mainly vibrate in place
- Strong intermolecular forces – particles held in fixed positions
- Fixed structure – very limited movement possible
Examples:
- Metal atoms diffusing at high temperatures
- Impurities spreading through crystals
- Gas diffusion through polymer membranes
Factors Affecting Rate of Diffusion
Temperature Effect
Higher Temperature = Faster Diffusion
- Increased kinetic energy → particles move faster
- More energetic collisions → particles spread more rapidly
- Greater thermal motion → higher probability of movement
Mathematical Relationship: Average kinetic energy ∝ Temperature (in Kelvin)
Concentration Gradient Effect
Steeper Gradient = Faster Diffusion
- Greater difference in concentration → stronger driving force
- More particles available to move → higher diffusion rate
- Larger statistical imbalance → faster equilibration
Physical Barriers
Fewer Obstacles = Faster Diffusion
- Porous membranes allow faster diffusion than solid barriers
- Larger pores permit faster movement
- Thinner barriers reduce diffusion distance
.

Vapours ( Gas) —- Rain (Liquid)
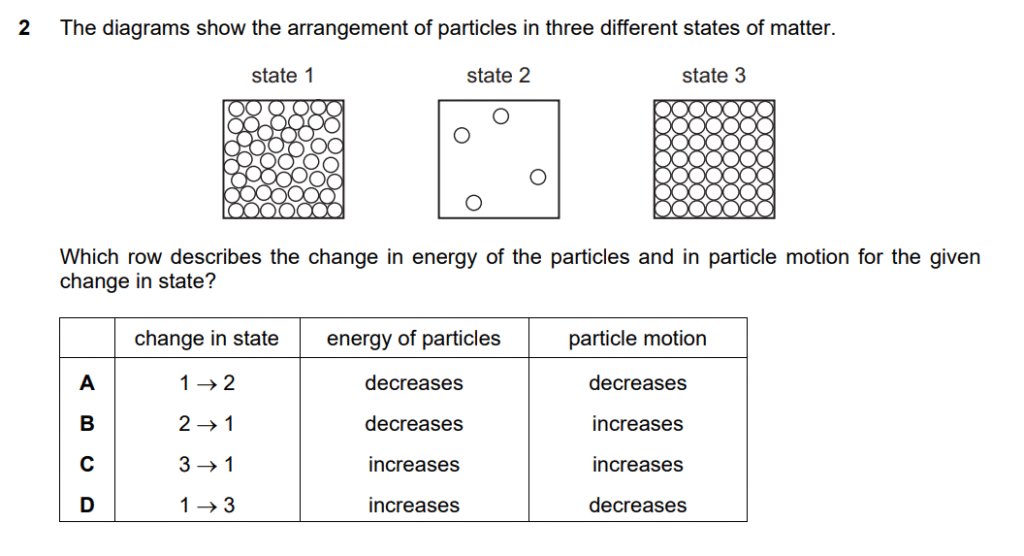
Step 1 : Liquid
Step 2 : Gas
Step 3 : Solid

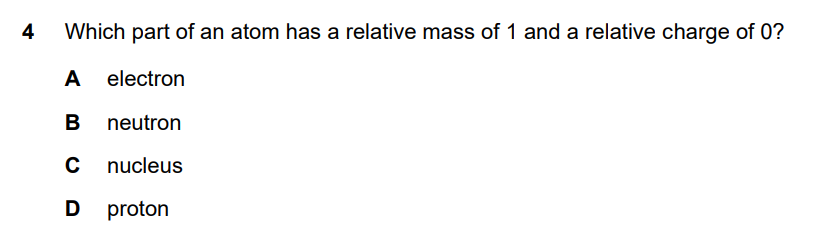
| Particle | Relative Mass | Relative Charge | Location |
|---|---|---|---|
| Proton | 1 | +1 | Nucleus |
| Neutron | 1 | 0 | Nucleus |
| Electron | 1/1836 (≈0) | -1 | Electron shells |
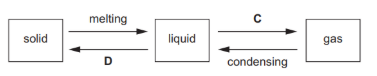
Name the changes of physical state
C : change from liquid to gas, which is called boiling or evaporation.
D : change from liquid to solid, which is called freezing or solidification.
