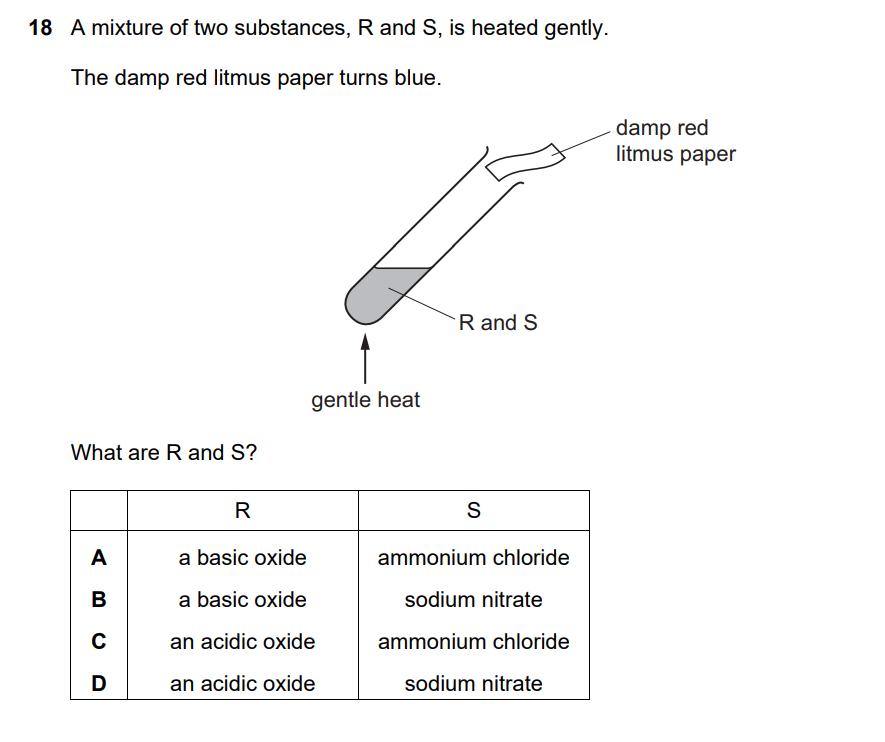
Key Information:
- Damp red litmus paper turns blue when heated with the mixture
- Red litmus turning blue indicates the presence of a base/alkali
Analysis:
- What causes litmus to turn blue?
- Ammonia gas (NH₃) – released when ammonium salts are heated
- Basic oxides can also cause this, but they’re typically solid
- Which combinations produce ammonia gas when heated?
- Ammonium chloride (NH₄Cl) when heated: NH₄Cl → NH₃ + HCl
- This happens regardless of what it’s mixed with
- Checking options:
- A & B: Basic oxide + NH₄Cl → Will release NH₃ gas ✓
- C & D: Acidic oxide + NH₄Cl → Will still release NH₃ gas ✓
- Options with sodium nitrate: No ammonia production ✗
- Most likely answer: Since the question emphasizes the litmus turning blue and both A and C contain NH₄Cl (which definitely produces NH₃), we need to consider which is more typical.
Answer: A
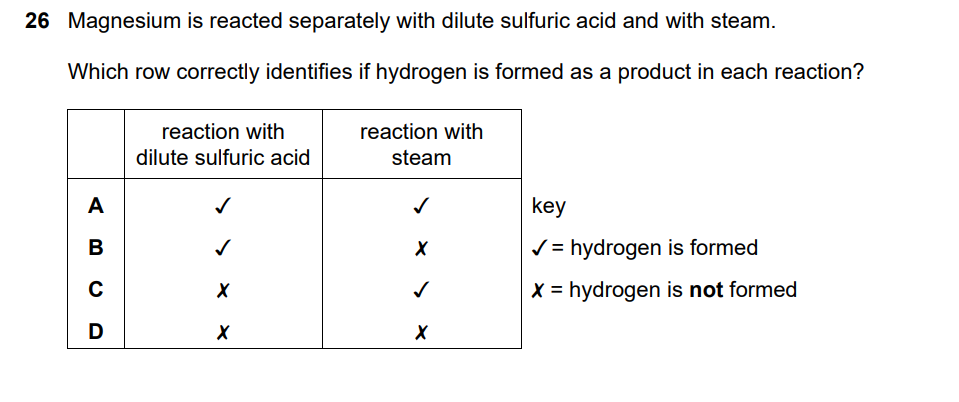
Reaction 1: Magnesium + dilute sulfuric acid
- Equation: Mg + H₂SO₄ → MgSO₄ + H₂
- Analysis: Metal + acid → salt + hydrogen gas
- Hydrogen formed? YES ✓
Reaction 2: Magnesium + steam
- Equation: Mg + H₂O(g) → MgO + H₂
- Analysis: Reactive metal + steam → metal oxide + hydrogen gas
- Key point: Steam (water vapor) at high temperature allows this reaction
- Hydrogen formed? YES ✓
Understanding the reactions:
- With dilute acid: Standard metal-acid reaction produces hydrogen
- With steam: Hot steam provides enough energy for magnesium to extract hydrogen from water molecules
Both reactions produce hydrogen gas because:
- Magnesium is reactive enough to displace hydrogen from both acids and steam
- Steam reaction requires higher temperature than acid reaction
Checking options:
- A: ✓ (acid), ✓ (steam) – Both produce hydrogen
- B: ✓ (acid), ✗ (steam) – Incorrect about steam
- C: ✗ (acid), ✓ (steam) – Incorrect about acid
- D: ✗ (acid), ✗ (steam) – Both incorrect
Answer: A
