
A: Elements arranged in order of increasing relative atomic mass
- Modern periodic table is arranged by atomic number (protons), not atomic mass
- Historical note: Mendeleev used atomic mass, but this caused problems
- Example: Ar (39.9) comes before K (39.1) by mass, but K comes first by atomic number ✗
B: Reactivity in Group I and Group VII increases down the groups
- Group I (alkali metals): Li → Na → K → Rb → Cs
- Reactivity increases down (easier to lose outer electron) ✓
- Group VII (halogens): F → Cl → Br → I
- Reactivity decreases down (harder to gain electron) ✗
- Statement claims both increase – this is incorrect ✗
C: Elements in same period have similar chemical properties
- Period = horizontal rows
- Elements in same period have different properties (Na, Mg, Al, Si, P, S, Cl, Ar)
- Groups (vertical columns) have similar properties, not periods ✗
D: Elements in Group II form ions with 2+ charge
- Group II = alkaline earth metals (Be, Mg, Ca, Sr, Ba)
- All have 2 outer electrons → lose both → form M²⁺ ions
- Examples: Mg²⁺, Ca²⁺, Ba²⁺ ✓
Answer: D
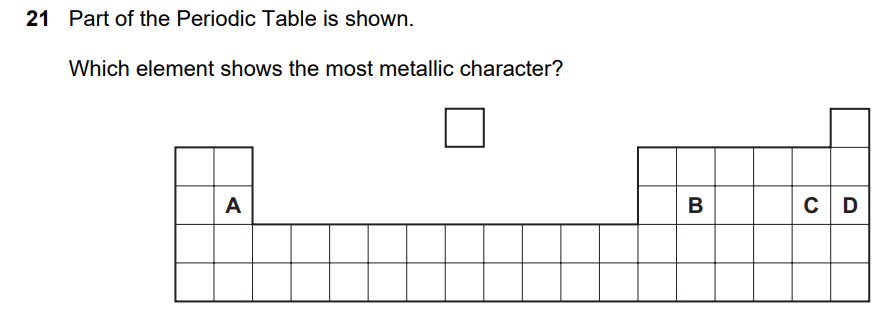
Metallic character increases:
- Down a group (easier to lose electrons as outer electrons are further from nucleus)
- Left to right across periods (fewer outer electrons to lose)
Analyzing positions from the diagram:
Position analysis:
- A: Upper left position – Group 2, earlier period
- B: Lower left position – Group 3, later period
- C: Lower middle-right position – Group 6/7, later period
- D: Upper right position – Group 8, earlier period
Metallic character ranking:
- B – Furthest down and towards the left = most metallic
- A – Left side but higher up
- D – Right side but higher up
- C – Right side and lower down = least metallic
Key principle: Elements become more metallic as you move down and left in the periodic table because:
- Larger atomic radius makes it easier to lose outer electrons
- Fewer outer electrons to lose
Answer: B

Group I elements (alkali metals): Li, Na, K, Rb, Cs, Fr
Key trends down Group I:
- Melting point decreases: Li > Na > K > Rb > Cs > Fr
- Density increases: Li < Na < K < Rb < Cs < Fr
Given constraints for element E:
- Higher melting point than caesium (Cs)
- Lower density than sodium (Na)
Applying constraints:
Melting point constraint: E has higher m.p. than Cs
- Cs has lowest m.p. in Group I
- So E could be: Li, Na, K, or Rb ✓
Density constraint: E has lower density than Na
- Na is second lightest in Group I
- Only lithium has lower density than Na ✓
Cross-checking lithium:
- Li melting point (181°C) > Cs melting point (28°C) ✓
- Li density (0.53 g/cm³) < Na density (0.97 g/cm³) ✓
Eliminating other options:
- K, Rb, Cs: All have higher density than Na ✗
- Fr: Radioactive, highest density ✗
Answer: A (lithium)
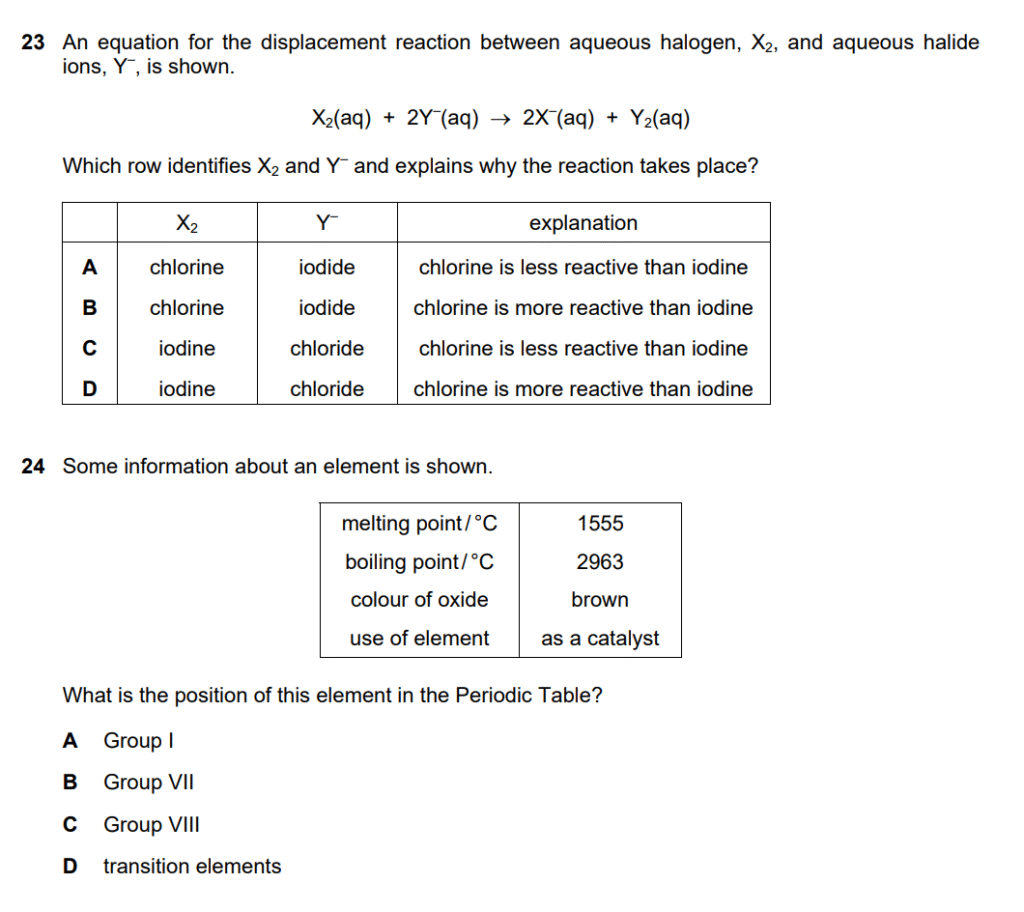
Understanding displacement reactions:
- More reactive halogen displaces less reactive halogen from its compound
- Halogen reactivity order: F₂ > Cl₂ > Br₂ > I₂ (decreases down Group VII)
Analyzing the equation: X₂(aq) + 2Y⁻(aq) → 2X⁻(aq) + Y₂(aq)
Key insight: X₂ displaces Y⁻, so X₂ must be MORE reactive than Y₂
Checking options:
- A & B: Cl₂ + I⁻ → Cl⁻ + I₂
- Chlorine is more reactive than iodine ✓
- Explanation should state “chlorine is more reactive than iodine”
- C & D: I₂ + Cl⁻ → I⁻ + Cl₂
- This would NOT occur (iodine less reactive than chlorine) ✗
Answer for Q23: B
Question 24 Analysis:
Given properties:
- High melting point (1555°C) and boiling point (2963°C)
- Brown oxide
- Used as catalyst
Analyzing each group:
A: Group I (alkali metals)
- Low melting/boiling points
- White/colorless oxides ✗
B: Group VII (halogens)
- Low melting/boiling points (except I₂)
- Not typically catalysts ✗
C: Group VIII (noble gases)
- Very low melting/boiling points
- Don’t form oxides readily
- Unreactive ✗
D: Transition elements
- High melting/boiling points ✓
- Colored oxides (often brown/black) ✓
- Commonly used as catalysts ✓
- Examples: Fe₂O₃ (brown), CuO (black)
Answer for Q24: D
