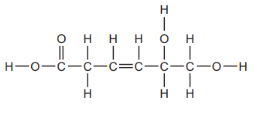
Identify Carboxylic Functional Group
Deduce the Molecular Formula :
C6H8O4
Explain why this compound is Unsaturated?
Compound is unsaturated because it contains carbon-carbon double bonds (C=C). Saturated compounds only have single bonds.
Write test ofr Unsaturated Compounds
Test: Add bromine water (orange-brown solution) to compound.
Observations: The orange-brown color of the bromine water will decolorise or turn colorless.
Alkanes are Hydrocarbons
State type of bonding in alkanes
Alkanes have covalent bonds. Specifically, all the bonds within an alkane molecule are single covalent bonds.
Ethane reacts with Chlorine in a substitution reaction. Draw structure
State the meaning of the term Hydrocarbon.
A hydrocarbon is an organic compound made up of only hydrogen atoms and carbon atoms.
Petroleum contain Hydrocarbons
Name the process used to separate petroleum into useful components.
Fractional distillation.
Name Uses
Gasoline/Petrol Used in Cars
Kerosene Fuel for Jets
Bitumen To surface roads
